Silver is quite different from gold because it is nearly always found within ores that require specialized mining techniques to extract. Silver ore is one of the most important resources on Earth. After processing and refining silver is used for countless industrial uses today.
The Main Sources and Uses for Silver
Silver is one of the most valued precious metals in the world. It is a key player in the world’s monetary systems mainly being used to create bullion coins. Other than its use in currencies, silver also finds wide application in the creation of solar panels, jewelry, utensils, electrical conductors, water filtration, window coatings and mirrors among other things. Silver is also used the medical filed as disinfectants, in x-ray machines and other medical instruments.
Silver is a soft white metallic element represented by the symbol Ag and atomic number 47. The element is known to exhibit the highest reflectivity, thermal and electrical conductivity of any known metal. Silver is usually found in the crust of the earth either as a free element (native silver) or more commonly as an alloy of gold or other metallic elements.
It is not very abundant in native form and thus its purity is measured using a per mille measurement. In many places, silver ore is mined as a byproduct of gold, copper, zinc, or lead.
Characteristics of Silver
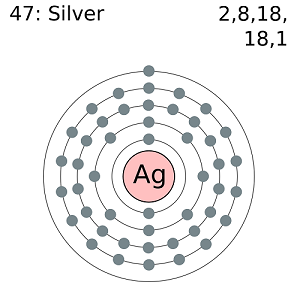 Silver has the same physical and chemical characteristics as its two group 11 neighbors in the periodic table: gold and copper.
Silver has the same physical and chemical characteristics as its two group 11 neighbors in the periodic table: gold and copper.
Silver is a somewhat inert metal. This is on account of its filled 4d shell is not extremely powerful in protecting the electrostatic powers of attraction from the core to the outermost 5s electron. Among all the group 11 elements, silver has the most minimal first ionization energy, yet has higher second and third ionization energies than copper and gold.
It must be noted in spite of the above characteristics most silver compounds have more covalent character because of the high first ionization vitality (730.8 kJ/mol) and the small size of silver. Furthermore, silver’s Pauling electronegativity of 1.93 is higher than that of lead (1.87), and its electron proclivity of 125.6 kJ/mol is much higher than that of hydrogen (72.8 kJ/mol) and very little not as much as that of oxygen (141.0 kJ/mol).[24] Due to its full d-subshell, silver in its principle +1 oxidation state displays a few of properties of the transition metals appropriate from groups 4 to 10, forming rather unstable organometallic compounds.
Silver just like gold and copper is soft malleable and extremely ductile. Silver characteristically crystallizes into a face-centered cubic lattice with mass coordination number 12, where just the single 5s electron is delocalized, just like the case of copper and gold. Metallic bonds in silver are inadequate with regards to a covalent character and are moderately weak. This helps explain the ductility and high malleability of silver.
Silver has very high electrical conductivity even when compared to copper and that is why it is widely applied in radio frequency engineering where high electrical conductivity is desired. Copper is widely used for most other application because the higher cost of silver often limits its use. Silver also has the highest thermal conductivity and the lowest resistance of any given metal.
Silver Mining Process
 Silver is mined using a number of processes. One of the most common processes of extracting silver metal for the ore is the heap leach or cyanide process. The process is most popular with many miners because it is low cost, especially when processing low-grade ores.
Silver is mined using a number of processes. One of the most common processes of extracting silver metal for the ore is the heap leach or cyanide process. The process is most popular with many miners because it is low cost, especially when processing low-grade ores.
To use the cyanide process the silver being in the ore should have smaller particles, should be able to react with the cyanide solution, the silver should be free from sulfide minerals and other foreign substances. The following are the major steps involved in silver mining using this method:
Preparation of Silver Ore
The first stage of mining silver involves the crushing the silver ore to about 1-1.5in diameter so as to make the ore porous for the extraction process. Once the ore is crushed it is then mixed with lime (about 3-5 lb. per ton) to create a conducive alkaline conditions for the extractions. The ore is then stacked onto impermeable pads made of asphalt, rubber or plastic to ensure that there is a minimum loss of the silver cyanide solution once the extraction begins. Usually, the pads are arranged in a slanting position to allow for the drainage and the collection of the silver cyanide solution.
Curing with the Cyanide solution
The next step is to add a solution of sodium cyanide and water to the prepared silver ore. Usually, this may be done using a number of methods such as sprinkler systems or ponding method that involves seepage from capillaries, ditches or injection.
Recovering the Silver

Once the curing is done the silver cyanide solution has to be collected so that the silver can be extracted from the solution. The most commonly used method of recovering silver from the solution is by Crowe Precipitation. This method employs zinc dust to help precipitate silver from the solution. The silver collected is then filtered off then melted and then made into bullion bars.
The other method used to recover silver from the silver cyanide solution is the activated carbon absorption method in which the solution has to be pumped through towers with activated carbon to form a silver precipitate. The precipitate is then collected by filtration and melted before it is made into bars.
In many places, silver ore is often found in combination with other ores containing other commercially viable minerals such as copper, lead or gold. When silver is a byproduct of processing these other minerals then a different method has to be used to extract the silver ore. For example is silver is found in ore containing zinc a method known as the Parkes Process is used to mine the minerals. When using this method the ore is first heated until it melts. When the ore is the cooled down a crust containing silver and zinc forms on the surface. The crust is then collected and then distilled to extract silver from the zinc.
When silver is found in ores containing copper then the electrolytic refining method is used to extract the silver. The ore is placed in an electrolyte solution with a cathode and an anode. Electricity is then passed through the solution forcing the silver to accumulate around the anode while copper attaches itself on the cathode. This is then collected and leached to remove impurities.

Silver Mining around the World
Silver is naturally found as an alloy with gold or in ores that contain chlorine, arsenic, sulfur and antimony. Major Ores include chlorargyrite (AgCl), argentite (Ag2S) and pyrargyrite (Ag3SbS3).
The major sources of silver are lead ores, copper ores, lead-zinc ores and copper-nickel ores found mostly in Mexico, Australia, Bolivia, Serbia, Peru, Chile, China, and Poland. Silver is produced mainly as a byproduct of the electrolytic refining of gold, nickel, copper and zinc in the ores. In some places it is produced by the Parkes Process used to refine lead bullion. Silver meant for commercial purposes must be at least 99.9% pure.
 Mexico, which was the leading silver producer in the world in 2015, has produced silver since 1546. The top five silver producers in the world include Mexico, Peru, Australia, Bolivia, and China.
Mexico, which was the leading silver producer in the world in 2015, has produced silver since 1546. The top five silver producers in the world include Mexico, Peru, Australia, Bolivia, and China.
The highest silver producing mines as of 2015 included the Rudna Mine in Poland, the Antamina Mine in Peru, the San Cristóbal Mine in Bolivia, the Penasquito Mine in Mexico, the Cannington Mine in Australia and the Fresnillo Mine in Mexico.
Some of the major mine development in the world expected to become top producers of silver ore include Malku Khota in Bolivia, Hackett River in Canada, Jaunicipio in Mexico, Pascua Lama in Chile and Navidad in Argentina.
Tajikistan in central Asia is considered to be one of the world largest silver deposits is also still under development and is has the potential to become a leading silver producer in the world.
Silver Production in the U.S.
Silver mining became a major commercial activity in 1858 following the discovery of silver deposit in the Comstock Lode in Nevada. Silver became a major mineral in the United States in 1873 when it was demonetized by the Coinage Act of 1873. Silver continued to play an important role in the United States especially during both the first and the second World Wars.
In 2014 about 1,170 tons of silver were produced in the United States. This constituted to just about 17% of the silver used in the same year in the country. The deficit was filled by imports from Mexico, Peru, Chile, and Canada. Silver is mined in several states across the United States.
The following are the top states mining for silver in the United States.
Alaska
Alaska was the top silver producer in the United States in 2015. The major silver producing mines in the state include the Hecla Mining’s Greens Creek mine which produced about 8,452,150 troy ounces of silver minerals in 2015 and the Red Dog Mine owned by Tech Resources and which is the world’s top zinc producer which produced about 7.6 million troy ounces of silver in the same year.
Idaho
Idaho is another great silver-producing state in the U.S. The Silver Valley (Coeur d’Alene District) is the highest silver producing district in the United States and one of the three in the world. The two largest silver producing mines in 2015 in the state were the Lucky Friday Mine which produced about 3 million troy ounces of silver and the Galena Mine which produced about 1.54 million troy ounces of silver.
Montana
Montana is another renowned silver state in the United States. The Butte district is only second to Coeur d’Alene when it comes to historical gold production. Silver was first discovered in Montana in 1864 and the state has contributed greatly to the metal production in the United States over the years.
Nevada
Nevada is where silver was first discovered in the United States in 1858 at the Comstock Lode. Silver was then discovered in several other areas within the state such as the Pioche (1869), Eureka (1864), El Dorado Canyon (1861), and Austin (1862) among others. Today, Nevada is home to the second largest and most productive silver mine in the United States. The Rochester Mine produced about 4.6 million ounces of silver in 2015 down from 6.7 ounces of silver 2014.
Also Read: This Nevada Gold Mine Produces Amazing Crystalline Gold Nuggets!
Utah
Silver in Utah was discovered in the late 1960s with the first discovery being within the Park City District. Some of the most famous mines in the state include the Ontarios Mine, the Silver King Mine, and the Flagstadd Mine. Today silver in the state is mainly a byproduct of copper and zinc mining. The highest silver mine in the state currently is the Bingham Canyon Mine.